
Artwork of a pair of woolly mammoths (Mammuthus primigenius) engaged in a fight for territory or status. (© Alamy)
Once the stuff of science fiction, and numerous false-starts, at last the extraction of bona fide ancient DNA from sediments (sedaDNA) has arrived. In contrast to DNA recovered from biological tissues such as bones and teeth, sedaDNA is extracted from sediments on the ocean floor, in soils, lakebeds or cave deposits. Genetic material in the skin, hair, faeces, urine, pollen or decaying tissues shed from organisms into their environment can bind to sediment particles and persist for hundreds of thousands and even millions of years. Analysis of this ancient DNA can aid reconstruction of past ecosystems and environmental change. For example, scientists have used sedaDNA to show that woolly mammoths lived in the Arctic long after they were thought to be extinct in the region (Graham et al., 2016), and to identify 2-million-year-old plant species in Greenland (Kjær et al., 2022).
So, what is possible using sedaDNA and, equally important, what is not? Do all sediments contain ancient DNA, and if not, why not? What are the potential gains in Earth science, and can we add sedaDNA to the list of microscopic remains that can be preserved in suitable sediments or rocks? What information can sedaDNA bring above and beyond the fossil remains of tissues of plants and animals, microfossils and other biomarkers. In short, is sedaDNA going to be revolutionary in the geosciences?
What is possible using sedaDNA and, equally important, what is not? Is sedaDNA going to be revolutionary in the geosciences?
Conditions for preservation
DNA is fragile, so to preserve sedaDNA over vast timescales requires specific, highly stable conditions, with low temperatures, low oxygen and moisture levels, low acidity and minimal sediment disturbance. The extraction of ~2-million-year-old sedaDNA from Greenland, specifically from the upper foreshore silty sands of the Kap København Formation (Kjær et al., 2022), therefore marked a major milestone. The oldest DNA extraction to date, this discovery implies that, under the right conditions, sedaDNA can be preserved for almost the entire Pleistocene epoch – and potentially even longer, if conditions allow.
The Kap København Formation case study also provides insight into the potential mechanisms for sedaDNA preservation, and particularly the importance of mineralogy.
Clay minerals are known to have a high affinity for DNA. Indeed, clays are implicated in theories of organic evolution because clay minerals can facilitate the replication of amino acids and potentially influence the evolution of metabolism. DNA can bind to clay minerals, or even become entrapped (nanoconfinement), offering protection from degradation. The Kap København sediments are rich in the swelling clay mineral smectite. This mineral has been shown to preserve sedaDNA in archaeological sediments even from the warm Mediterranean region, implying that when adequately protected from degradation, sedaDNA can persist in environments above the 10°C mean annual temperature isotherm that had been thought limiting for preservation.
DNA: The Basics

Conceptual illustration of a DNA molecule double helix – the DNA ‘ladder’ (© iStock)
DNA serves as an instruction manual for how living things grow and function. It is made up of four chemical building blocks, or bases, Adenine (A), Thymine (T), Cytosine (C) and Guanine (G) that form pairs to build a double helix – the DNA ‘ladder’. The order, or sequence, of these bases is unique to each living thing. To make it easier to identify the sequence, scientists must make multiple copies or amplify a specific section of the DNA. A Polymerase Chain Reaction (PCR) is a molecular biology technique used to replicate and amplify specific DNA sequences. A primer, a short piece of DNA, is used to mark the start point of the section of DNA to be copied and amplified. Typically, a synthetic enzyme is used as the primer.
Approach
Undoubtedly, the majority of DNA shed into the environment is destroyed by micro-organisms. The half-life of free DNA in water, for example, is measured in minutes or, at most, hours. But research is now showing that if a minute fragment of the DNA becomes adsorbed or incorporated into a mineral structure, then it can be preserved. Fragments of sedaDNA are relatively short, generally under 200 base pairs long (the rungs of the DNA ladder), making it difficult to identify the DNA and classify the organism (that is, to assign a name at a specific taxonomic level such as a family or species). Typically, with environmental DNA sequences, an enzyme is attached (a primer) allowing replication of the sequence using polymerase chain reactions (PCR) in what is known as a metabarcoding approach. Unfortunately, the primers used for environmental DNA are too long to be used for sedaDNA so Pierre Taberlet and colleagues developed a series of primers that could be used on short sequences (Taberlet et al., 2018).
An alternative approach to the metabarcoding method is the ‘shotgun’ approach whereby all the fragments are mapped against reference sequences and, in an allied methodology, multiple longer strands of DNA are broken into random smaller fragments. A computer algorithm is then used to reconstruct the original DNA sequence. This approach can, in theory, sequence all the DNA fragments in a sample. An early version employing cloning was used by Willerslev and colleagues (2003) to detect mammal and plant DNA preserved in Pleistocene permafrost sediments from Siberia and cave sediments from New Zealand. Under optimal conditions, the shotgun sequencing approach could allow the determination of ancestral groups (halpogroups), that is, shared genetic markers passed down by a common ancestor, thereby allowing reconstruction of evolutionary and migratory patterns in response to (for example) changes in climate and, in addition, estimates of population size.
There are advantages and disadvantages to both the metabarcoding and shotgun approaches. However, both can only identify sequences that are already known from DNA databases to which the sequences can be matched. Several botanical projects have created databases of distinctive sections of the plant, animal or microbe genomes in specific regions that aid identification. These databases include PhyloNorway (Tromsø, Norway), which is focused on Arctic-boreal vascular plants from Norway and polar regions, and PhyloAlps (Grenoble, France), which is focused on flora in the European Alps. Outside these regions analysis must rely on global databases. One day (and potentially quite soon) we will have the full genomes of all plant and animals sequenced. Numerous major international projects, such as the International Barcode of Life, Darwin Tree of Life and Earth BioGenome Project, are attempting this.
Geology, and particularly stratigraphy, are critical to the interpretation of sedaDNA. Currently, sedaDNA cannot be directly dated, so its value depends entirely on geological chronology, generally based on radiocarbon or luminescence dating methods, the fossil record, or amino acid racemization (a process whereby amino acids in living organisms convert from their L-form to a D-form over time at a predictable rate after death, thereby serving as a time marker).
New vistas
So, what is actually being done with this new tool and what are its potential applications? The answer is: too much to detail here, but I will highlight a few examples.
SedaDNA offers the ability to use the sedimentary archives to track the extinction of animals. So, instead of having to rely on the chance recovery of the bone of the last-surviving mammoth, which is an unlikely find, several sedimentary archives can be used to reliably estimate the timing of an extinction or local eradication. Researchers at the Paleogenomics Laboratory at Santa Cruz, USA, for example, were able to show that the last mammoth on the island of St Paul’s in the Bering Staits probably died c. 5,650±80 years ago (Graham et al., 2016) – well before humans arrived in the 18th century.

Illustration of woolly mammoths in a winter landscape © iStock
We can also see in real-time the processes of colonisation of the formerly glaciated parts of the globe, measure the rates of species immigration and establishment, and identify the critical controls, including the role of plants themselves – findings that have important implications for how both plants and animals will respond in a rapidly warming world).
Work by researchers at the Tromsø Museum laboratories in Norway is comparing the sedaDNA archive to geological data provided by the fossil record, as well as the archaeological record created by ancient humans who drew animals on rock-surfaces at over a thousand locations in Scandinavia. As with so much of geology, the triangulation of techniques can greatly increase confidence in our results. Instead of guessing or estimating from the historical record (which can be in error), we can directly specify if a particular animal or plant really did exist at that location prior to the radical alteration of Earth’s surface caused by, for example, agriculture and industrialisation. Such insights are important for conservation and resource management, providing evidence to inform our re-wilding or ecological restoration efforts, as well as answers to questions around the native versus non-native status of species.
In the marine environment, analysis of fossil foraminifera, ostracod or other eukaryote faunas retrieved from continental shelf and marine basin sediments are critical for tracking past ocean dynamics, calculating past sea surface temperatures and even past oceanic productivity. SedaDNA analysis could further allow us to reconstruct basin isolation, sea-ice distribution or methane-generation from gas hydrates.
SedaDNA also offers the intriguing possibility of looking at evolution in real time, even for organisms that at best only leave trace fossils. The most obvious examples are the annelids (worms) that, as Darwin observed, are critical elements in soil processes, yet leave no direct trace. Because of their high biomass (also observed by Darwin), worms are well represented in both soil and lake DNA profiles, and so sedaDNA offers the potential to understand the long-term dynamics and evolution of these incredibly important creatures for the first time.
The analysis of sedaDNA is providing new avenues in ‘geoarchaeology’ – the application of geological techniques to archaeological questions. Caves, it turns out, are great environments for the preservation of DNA due to their stable conditions and the presence of DNA-holding minerals. In a landmark study, the archaeogenetic group at the Max Planck Evolutionary Anthropology Laboratory in Leipzig, Germany, analysed resin-impregnated, thin section slides of cave sediments collected over the past 40 years from prehistoric sites across Europe, Asia, Africa and North America (Massilani et al., 2021) revealing evidence for cave-dwelling animals (many now extinct) and the presence of Palaeolithic humans. The researchers showed that DNA from bone and fossilised animal faeces (coprolite) was preserved in the cave sediments despite the exothermic processes required to create the thin-section slides used for optical microscopy. This finding turns the vast soil and sediment thin-section archive into a potential sedaDNA archive with implications for generations of geological studies on archaeological sites.
sedaDNA is providing new avenues in geoarchaeology
Ongoing studies on museum collections themselves are also revealing that many artifacts, even some collected many years ago, can still contain ancient DNA. These include skins, daub, and pottery. Interestingly, contamination might also be used to reveal the history of handling and even transportation of these artifacts.
Studies in cold environments have also shown that middens (historical refuse heaps) preserve sedaDNA well, greatly expanding estimates of past dietary breadth, and effectively doubling the list of species identified using the fossil record alone. Similar results are also now appearing from middens and refuse-rich sediments from much warmer environments (where the mineralogy is suitable) including shallow marine or fluvio-marine sediments that have silted up so many Greek and Roman ports in both the Mediterranean and southern Britain (part of a new project at the University of Southampton, in conjunction with the Southampton Marine and Maritime Institute).
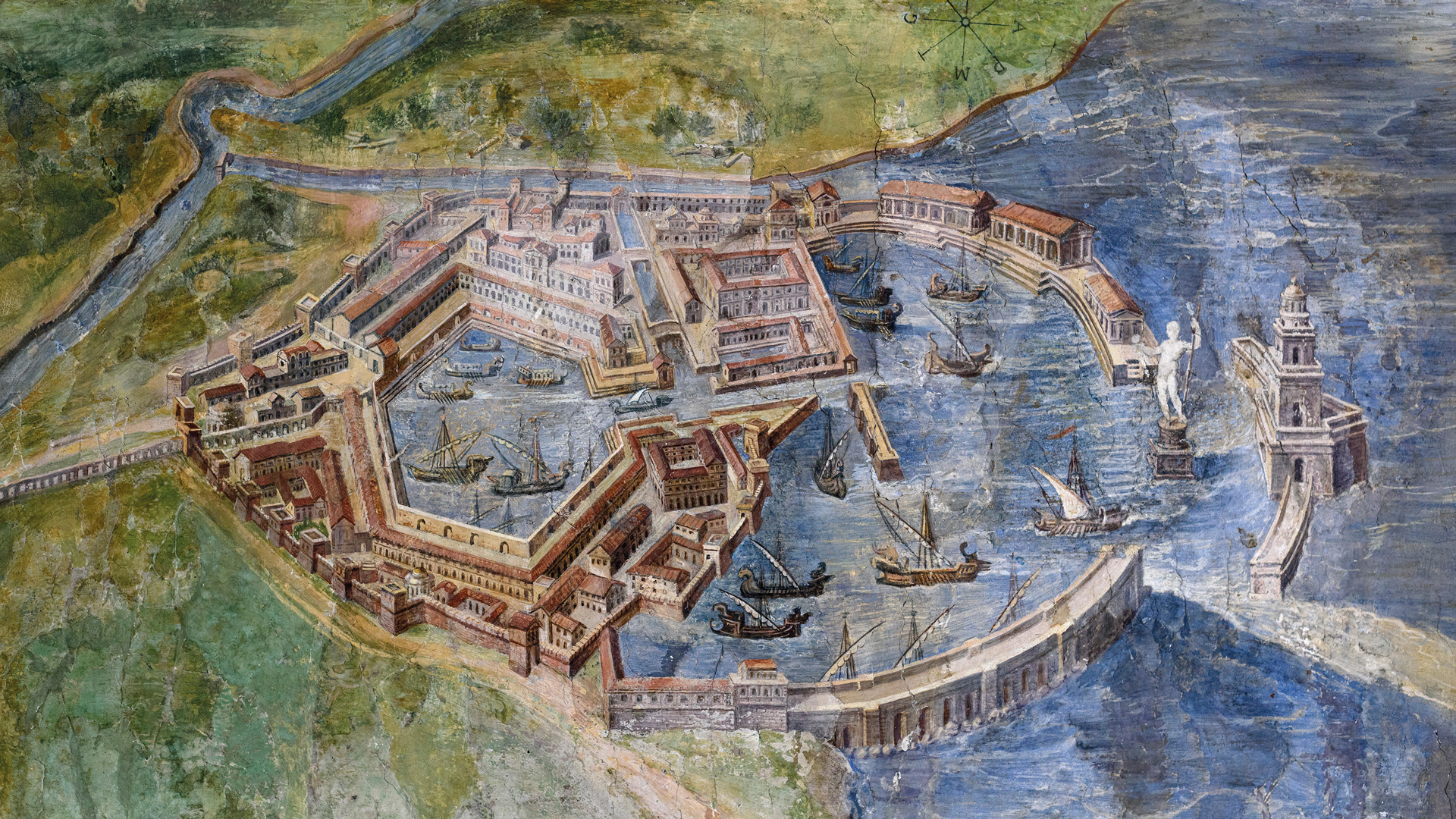
Painting of Portus, an artificial harbour established by Claudius, near the port of Ostia, Rome, Italy. (© Alamy)

Port harbours, which offer a unique window into population, diet, health, technology, and environmental change, may also be traps for sedaDNA. The Ancient Ports of Europe: A Novel Genetic Window on Ancient Lives (PortGEN) project is using rigs to extract sediment cores from historic European and Mediterranean harbours, including Portus and Ostia – harbours that once served ancient Rome. (© PortGEN)
Finally, the sedaDNA revolution is opening up completely new vistas of geological research into the evolution and history of microscopic life, varying from host to pathogen, from multi-cellular organisms to bacteria and even viruses (see pull-out box, sedaDNA: Multitude of Applications). Such DNA sequences are difficult to identify because the vast majority of micro-organisms are un-sequenced, and the species concept itself is at its stretching point with asexual reproduction, gene sharing, and incorporation. However, sedaDNA offers a new window into the biotic component of biogeochemical processes that not only creates life but regulates and sustains it. Current studies concentrate on the well-known and economically important organisms such as soil bacteria involved in nutrient cycling, and pathogens of plants and animals. The ability to reconstruct not only the macroscopic flora and faunas but also the microfaunas and microbiome is on the road to what has been termed ‘full ecosystem analysis’ – almost certainly a Holy Grail but one that will drive innovation.
Limitations
SedaDNA is not without its pitfalls and limitations and the most serious of these are geological in nature. Not all sediments will contain enough DNA for meaningful identification due to unsuitable petrography, mineralogy or diagenesis. Even if there is enough DNA, problems can arise due to leaching in porous sediments (including soils), bioturbation by plants and animals, and geomorphic processes such as sediment erosion and redeposition. Indeed, the extent to which sediment-bound DNA can be reworked is largely unknown, but this is clearly a possibility just as it is with many other microfossils. Other limitations, such as incomplete reference databases, are fundamentally technical and will be resolved by the bewildering pace of research into ancient DNA.
sedaDNA: Multitude of Applications
- Climate change from bioindicators without the fossils (e.g. bivalve DNA from sediments)
- Parasites and host-parasite interactions back in time (hologenomics)
- Evolution and ecology of non-fossil organisms (e.g. worms)
- Tracing the lineages of both extant and extinct organisms
- Diet analysis of extinct and extant megaherbivores
- Resolving the extent of ‘dark’ biodiversity
- Micro-evolution in time and space
- Clays and the origins of life
- Carbon reservoir analysis
Boundless potential
There has been some disquiet amongst palaeontologists and palaeoecologists that DNA will make these areas of geology redundant, but this is most unlikely because where the species concept holds, we will always want to relate the ‘genotype’ to the ‘morphotype’. With sedaDNA, we one day may be able to say much more about the scales, processes and plasticity of evolutionary processes than we ever could before.
Despite the current hurdles, as a new field in the geosciences, sedaDNA offers remarkable potential, opportunity, and numerous avenues of research that are fundamental to Earth science. SedaDNA offers the potential to reconstruct past ecosystems and biodiversity in unprecedented detail and could revolutionise not only our understanding of past environments, but also how ecosystems may respond to climate change in the future.
Author
Prof Tony Brown, Professor in Physical Geography, University of Southampton, UK
Further reading
- Alsos, I.G. et al. (2024) Using ancient sedimentary DNA to forecast ecosystem trajectories under climate change. Trans. R. Soc. B37920230017; http://doi.org/10.1098/rstb.2023.0017
- Brown, A.G. et al. (2025) The sedaDNA revolution and Archaeology: progress, challenges, and a research agenda. J. Archaeol. Sci. 174, 106132; https://doi.org/10.1016/j.jas.2024.106132
- Graham, R.W. et al. (2016) Timing and causes of mid-Holocene mammoth extinction on St. Paul Island, Alaska. Proc. Natl. Acad. Sci. U.S.A. 113 (33) 9310-9314; https://doi.org/10.1073/pnas.1604903113
- Kjær, K. H. et al. (2022) A 2-million-year-old ecosystem in Greenland uncovered by environmental DNA. Nature 612, 283–291; https://doi.org/10.1038/s41586-022-05453-y
- Massilani, D., et al. (2022) Microstratigraphic preservation of ancient faunal and hominin DNA in Pleistocene cave sediments. Proc. Natl. Acad. Sci. USA 119, e2113666118; https://doi.org/10.1073/pnas.2113666118.
- Murchie, T.J. et al. (2021) Collapse of the mammoth-steppe in central Yukon as revealed by ancient environmental DNA. Nat. Commun. 12, 7120; https://doi.org/10.1038/s41467-021-27439-6
- Taberlet, P. et al. (2018) Environmental DNA: For Biodiversity Research and Monitoring. Oxford Academic (Online edition); https://doi.org/10.1093/oso/9780198767220.001.0001
- Willerslev, E. et al., (2003) Diverse Plant and Animal Genetic Records from Holocene and Pleistocene Sediments. Science 300, 791–795; DOI: 10.1126/science.1084114
- The sedaDNA Society; https://sedadna.github.io/
Citation: Brown, A. From mammoths to worms. Geoscientist 35 (3), 28-33, 2025. DOI: 10.1144/geosci2025-022.











